
The experimental data further show that cancer-associated membrane transformations may account for mitochondria targeting by betulinic acid and resveratrol, known anti-cancer molecules. Specifically, isolation of cell mitochondria and preparation of inverted sub-mitochondrial particles (SMPs) illuminated significant cancer-induced modulations of membrane lipid compositions, fluidity, and activity of cytochrome c oxidase, one of the key mitochondrial enzymes. This study compares the structural and functional properties of mitochondrial membranes in prostate and colon cancer cells in comparison to normal mitochondria, and possible therapeutic implications of these membrane changes. However, the role of mitochondrial membranes in cancer onset and progression has not been thoroughly investigated. Mitochondria have emerged as important determinants in cancer progression and malignancy.

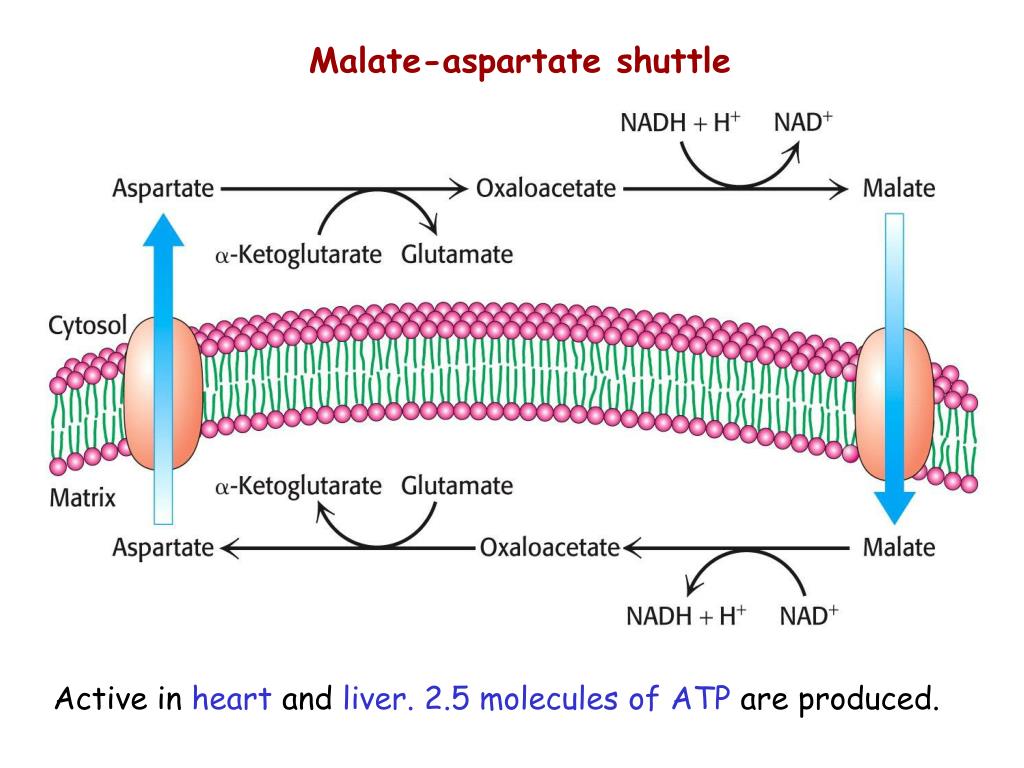
The mitochondrial malate–aspartate and lactate shuttles cooperate to enable aerobic oxidation of glycolytic l‐lactate in mitochondria. The elevated glycolysis in cancer cells is proposed to be one of the mechanisms acquired to accelerate oxidative phosphorylation. We demonstrated that the malate–aspartate shuttle exerts control over NAD+/NADH homeostasis to maintain activity of mitochondrial lactate dehydrogenase and to enable aerobic oxidation of glycolytic l‐lactate in mitochondria. We investigated the role of mitochondria‐associated malate–aspartate and lactate shuttles in colon cancer cells as potential regulators that couple aerobic glycolysis and oxidative phosphorylation. We hypothesized that activities of glycolysis and oxidative phosphorylation are coordinated to maintain redox compartmentalization. However, there are a growing number of studies that show that mitochondria remain highly oxidative under glycolytic conditions. Elevated glycolysis underlying the Warburg effect is an established signature of cancer metabolism. Therefore, many cancer types, including colon cancer, reprogram mitochondria‐dependent processes to fulfill their elevated energy demands. Within the context of these competing drives aerobic glycolysis is inefficient for the cancer cellular energy economy. Page generated by Pathway Tools version 19.5 (software by SRI International) on Fri Oct 14, 2022.Metabolism in cancer cells is rewired to generate sufficient energy equivalents and anabolic precursors to support high proliferative activity. "Biochemical characterization of stage-specific isoforms of aspartate aminotransferases from Trypanosoma cruzi and Trypanosoma brucei." Mol Biochem Parasitol 161(1) 12-20. Marciano08: Marciano D, Llorente C, Maugeri DA, de la Fuente C, Opperdoes F, Cazzulo JJ, Nowicki C (2008). "The malate dehydrogenase isoforms from Trypanosoma brucei: subcellular localization and differential expression in bloodstream and procyclic forms." Int J Parasitol 36(3) 295-307. References Related to Enzymes, Genes, Subpathways, and Substrates of this PathwayĪranda06: Aranda A, Maugeri D, Uttaro AD, Opperdoes F, Cazzulo JJ, Nowicki C (2006).

The reaction is unique to this pathway in MetaCyc This organism is not in the expected taxonomic range for this pathway.Īn enzyme catalyzing this reaction is present in this organism


 0 kommentar(er)
0 kommentar(er)
